Sum-frequency generation vibronic spectroscopy laboratory
- Surface science, catalysis, pressure and material gaps.
- The Vibronic Spectroscopy Laboratory.
- News and selected Research Highlights.
- Research Group.
- Former students/staff.
- Collaborations.
- Funding and contributors.
- Recent Relevant Publications.
- Back to Surface Science Group page
Surface science, catalysis, pressure and material gaps.

Nature performs catalytic reactions for the synthesis of energy vectors and organic compounds by exploiting nano-sized or single-atom photosystem and enzymatic catalytic centers, where metals (Mn, Cu, Ni, Fe…) are supported by C, S, or N linkers. Our research is focused on heterogeneous catalytic reaction mechanisms, investigated at the atomic level detail from UHV to near-ambient pressure conditions, occurring at single crystals, nanostructured surfaces, and model catalysts, with an extension to the electro-catalytic environment. The chemical, electronic, and structural properties are studied experimentally by means of surface science approaches, synchrotron radiation spectroscopies, and in situ and operando IR-Vis Sum-Frequency generation vibronic spectroscopy as implemented in the facility hosted by our lab. Fostering external collaborations, the group exploits ab initio simulations within the framework of Density Functional Theory to yield insight and proper interpretation of the experimental results.
The Vibronic Spectroscopy Laboratory
The Laboratory was designed in 2012 and commissioned in 2013 within the framework of the MIUR FIRB2010 project RBFR10J4H7. The installed spectrometer (Ekspla) allows for IR-Vis SFG vibronic spectroscopy investigation of solid-gas, solid-liquid, and liquid-gas interfaces. An IR pulsed laser beam (50 Hz, 30 ps, 1064 nm) is converted into visible (532 nm) and tunable IR (1500-4000 cm-1) radiation that is temporally and spatially overlapped at the sample’s surface. A monochromator and a CCD detector yield scanning-mode data collection. The polarization of the beams can be selected (p and s modes).
An ultra-high vacuum (UHV) system is coupled to the spectrometer. The base pressure of 7E-11 mbar allows for standard surface science sample preparation, pre- and post-analysis. The installed instrumentation includes an ion gun (Eurovac), a gas line, LEED optics (OCI), RGA, and fast-entry lock for sample loading. The sample can be resistively heated up to 1300 K. Liquid nitrogen cooling is available. Without breaking the vacuum, the sample can be transferred into a reaction cell that is directly aligned with SFG spectrometer (5 degrees of freedom manipulator). Impinging and outgoing light is transferred through BaF2 windows. Achievable reaction conditions in the measurement cell are in the 1E-10 – 1E+3 mbar and 300 – 1000 K ranges. Up to three different gas-phase reactants can be used in parallel.

An electrolytic cell is also available for electrochemistry measurements. The setup was developed in collaboration with CNR-ICCOM and UniSalento. Measurements in situ and operando can be performed at an electrode’s surface in the -5/+5 V range at the solid-liquid interface. Electrolyte’s recycling and gas bubbling devices are available. The optical configuration includes a CaF2 prism with micro-channels for the handling of both the electrolyte and the gas bubbles.
News and Selected Research Highlights
News.
- On the occasion of the Users Meeting, the M.Sc. Francesco Armillotta, XXXV cycle Ph.D. student in Physicsin our group, has been awarded the “MAX IV Students Science Award 2021” of the Max IV Swedish synchrotron in Lund
Research Highlights.
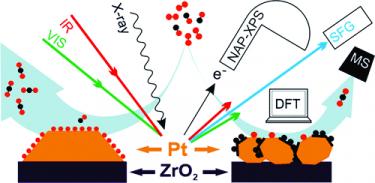 Interplay between CO disproportionation and oxidation: on the origin of the CO reaction onset on ALD-grown Pt/ZrO2 model catalysts [ACS Catal 2021]. Pt/ZrO2 model catalysts were prepared by atomic layer deposition (ALD) and examined at mbar pressure by operando sum frequency generation (SFG) spectroscopy and near ambient pressure X-ray photoelectron spectroscopy (NAP-XPS), combined with differentially-pumped mass spectrometry (MS). ALD enables creating model systems ranging from Pt nanoparticles to bulk-like thin films. Polarization-dependent SFG of CO adsorption reveals both the adsorption configuration and the Pt particle morphology. By combining experimental data with ab initio simulations within the framework of Density Functional Theory (DFT), we show that the CO reaction onset is determined by a delicate balance between CO disproportionation (Boudouard reaction) and oxidation. CO disproportionation occurs on low-coordinated Pt sites, but only at high CO coverages and when the remaining C-atom is stabilized by a favorable coordination. Thus, under the current conditions, initial CO oxidation is found to be strongly influenced by the removal of carbon deposits formed through disproportionation mechanisms, rather than being determined by the CO and oxygen inherent activity. Accordingly, at variance with the general expectation, rough Pt nanoparticles are seemingly less active than smoother Pt films. The applied approach enables bridging both the "materials and pressure gaps".
Interplay between CO disproportionation and oxidation: on the origin of the CO reaction onset on ALD-grown Pt/ZrO2 model catalysts [ACS Catal 2021]. Pt/ZrO2 model catalysts were prepared by atomic layer deposition (ALD) and examined at mbar pressure by operando sum frequency generation (SFG) spectroscopy and near ambient pressure X-ray photoelectron spectroscopy (NAP-XPS), combined with differentially-pumped mass spectrometry (MS). ALD enables creating model systems ranging from Pt nanoparticles to bulk-like thin films. Polarization-dependent SFG of CO adsorption reveals both the adsorption configuration and the Pt particle morphology. By combining experimental data with ab initio simulations within the framework of Density Functional Theory (DFT), we show that the CO reaction onset is determined by a delicate balance between CO disproportionation (Boudouard reaction) and oxidation. CO disproportionation occurs on low-coordinated Pt sites, but only at high CO coverages and when the remaining C-atom is stabilized by a favorable coordination. Thus, under the current conditions, initial CO oxidation is found to be strongly influenced by the removal of carbon deposits formed through disproportionation mechanisms, rather than being determined by the CO and oxygen inherent activity. Accordingly, at variance with the general expectation, rough Pt nanoparticles are seemingly less active than smoother Pt films. The applied approach enables bridging both the "materials and pressure gaps".
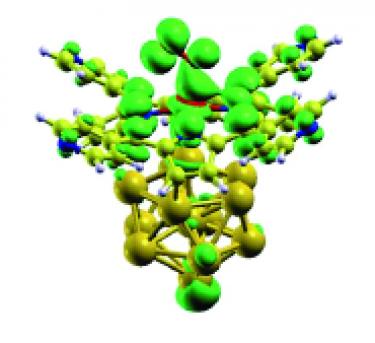 Stabilization and activation of molecular oxygen at biomimetic tetrapyrroles on surfaces: from UHV to near-ambient pressure [NanoscaleAdv 2021]. Recent advances in the development of surface science methods have allowed to bridge, at least partially, the pressure gap between the ultra-high vacuum environment and some applicative conditions. This step has been particularly critical for the characterization of heterogenous catalytic systems (solid-liquid, solid-gas interfaces) and, specifically, of the electronic, structural, and chemical properties of tetrapyrroles at surfaces when arranged in 2D networks. Within a biomimetic picture, in which 2D metalorganic frameworks are expected to model and reproduce in a tailored way the activity of their biochemical proteic counterparts, the fundamental investigation of the adsorption and activation of small ligands at the single-metal atom reaction sites has progressively gained increasing attention. Concerning oxygen, biology offers a variety of tetrapyrrole-based transport and reaction pockets, as e.g. in haemoglobin, myoglobin or cytochrome proteins. Binding and activation of O2 are accomplished thanks to complex charge transfer and spin realignment processes, sometimes requiring cooperative mechanisms. Within the framework of surface science at near-ambient pressure (towards and beyond the mbar regime), recent progress has unveiled novel and interesting properties of 2D metalorganic frameworks and heterostacks based on self-assembled tetrapyrroles, thus opening possible, effective applicative routes in the fields of light harvesting, heterogenous (electro-)catalysts, chemical sensing, and spintronics.
Stabilization and activation of molecular oxygen at biomimetic tetrapyrroles on surfaces: from UHV to near-ambient pressure [NanoscaleAdv 2021]. Recent advances in the development of surface science methods have allowed to bridge, at least partially, the pressure gap between the ultra-high vacuum environment and some applicative conditions. This step has been particularly critical for the characterization of heterogenous catalytic systems (solid-liquid, solid-gas interfaces) and, specifically, of the electronic, structural, and chemical properties of tetrapyrroles at surfaces when arranged in 2D networks. Within a biomimetic picture, in which 2D metalorganic frameworks are expected to model and reproduce in a tailored way the activity of their biochemical proteic counterparts, the fundamental investigation of the adsorption and activation of small ligands at the single-metal atom reaction sites has progressively gained increasing attention. Concerning oxygen, biology offers a variety of tetrapyrrole-based transport and reaction pockets, as e.g. in haemoglobin, myoglobin or cytochrome proteins. Binding and activation of O2 are accomplished thanks to complex charge transfer and spin realignment processes, sometimes requiring cooperative mechanisms. Within the framework of surface science at near-ambient pressure (towards and beyond the mbar regime), recent progress has unveiled novel and interesting properties of 2D metalorganic frameworks and heterostacks based on self-assembled tetrapyrroles, thus opening possible, effective applicative routes in the fields of light harvesting, heterogenous (electro-)catalysts, chemical sensing, and spintronics.
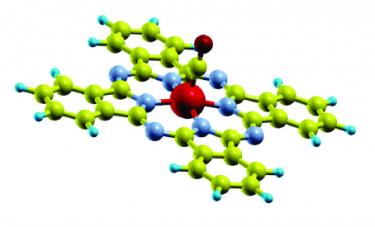
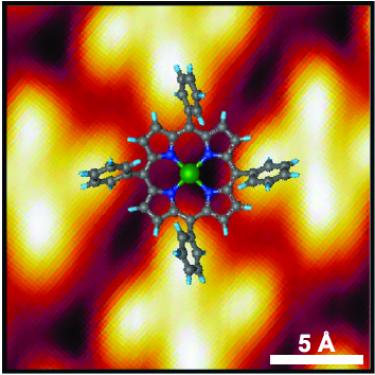 Room-temperature on-spin-switching and tuning in a porphyrin-based multifunctional interface [Small 2021]. Molecular interfaces formed between metals and molecular compounds offer a great potential as building blocks for future opto-electronics and spintronics devices. Here, we use a combined theoretical and experimental spectro-microscopy approach to show that the charge transfer occurring at the interface between Ni-tetraphenyl porphyrins and copper changes both spin and oxidation states of the Ni ion from [Ni(II), S=0] to [Ni(I), S=1/2]. The chemical active Ni(I), even in a buried multilayer system, can be functionalized with nitrogen dioxide allowing selectively tuning the electronic properties of the Ni center, that is changed to [Ni(II), S=1]. While Ni acts as a reversible spin switch, we find that the electronic structure of the macrocycle backbone, where the frontier orbitals are mainly localized, remains unaffected. Our findings pave the way for using the present porphyrin-based system as a platform for the realization of multifunctional devices where the magnetism and the optical/transport properties can be controlled simultaneously by independent stimuli.
Room-temperature on-spin-switching and tuning in a porphyrin-based multifunctional interface [Small 2021]. Molecular interfaces formed between metals and molecular compounds offer a great potential as building blocks for future opto-electronics and spintronics devices. Here, we use a combined theoretical and experimental spectro-microscopy approach to show that the charge transfer occurring at the interface between Ni-tetraphenyl porphyrins and copper changes both spin and oxidation states of the Ni ion from [Ni(II), S=0] to [Ni(I), S=1/2]. The chemical active Ni(I), even in a buried multilayer system, can be functionalized with nitrogen dioxide allowing selectively tuning the electronic properties of the Ni center, that is changed to [Ni(II), S=1]. While Ni acts as a reversible spin switch, we find that the electronic structure of the macrocycle backbone, where the frontier orbitals are mainly localized, remains unaffected. Our findings pave the way for using the present porphyrin-based system as a platform for the realization of multifunctional devices where the magnetism and the optical/transport properties can be controlled simultaneously by independent stimuli.
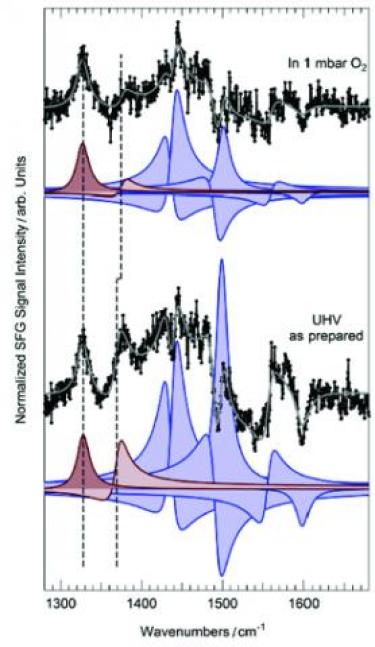 Self-metalation of porphyrins at the solid-gas interface [AngewChem 2021]. Self-metalation is one of the most promising synthesis routes to include a single metal atom in a tetrapyrrolic macrocycle in the case of organic frameworks supported by metal surfaces. The molecule-surface interaction may provide the charge transfer and the geometric distortion of the molecular plane that are necessary for metal inclusion, thus mimicking enzymatic metalation mechanisms of porphyrins in biology. However, while the latter process proceeds at room temperature, at a metal surface, instead, the presence of an activation barrier can represent a technical obstacle that cannot be compensated by a higher substrate temperature without affecting the layer integrity. The formation of the intermediate state, the sitting-atop complex that weakens the two N-H bonds in the tetrapyrrole preceding the metal insertion step, can be facilitated in some cases by oxygen pre-adsorption at the supporting metal surface, like in the case of the 2H-TPP/Pd(100) system. In such cases, the activation barrier can be overcome by mild annealing, yielding the formation of desorbing products (hydrogen, water) and of the metalated tetrapyrrole. We show here that the self-metalation of 2H-TPP molecules at the Pd(100) surface can be promoted already at room temperature by the presence of an oxygen gas phase at close-to-ambient conditions via an Eley-Rideal mechanism, resembling the asymmetric interactions available in enzymatic pockets, thus opening the way to novel routes to metalate organic frameworks at surfaces.
Self-metalation of porphyrins at the solid-gas interface [AngewChem 2021]. Self-metalation is one of the most promising synthesis routes to include a single metal atom in a tetrapyrrolic macrocycle in the case of organic frameworks supported by metal surfaces. The molecule-surface interaction may provide the charge transfer and the geometric distortion of the molecular plane that are necessary for metal inclusion, thus mimicking enzymatic metalation mechanisms of porphyrins in biology. However, while the latter process proceeds at room temperature, at a metal surface, instead, the presence of an activation barrier can represent a technical obstacle that cannot be compensated by a higher substrate temperature without affecting the layer integrity. The formation of the intermediate state, the sitting-atop complex that weakens the two N-H bonds in the tetrapyrrole preceding the metal insertion step, can be facilitated in some cases by oxygen pre-adsorption at the supporting metal surface, like in the case of the 2H-TPP/Pd(100) system. In such cases, the activation barrier can be overcome by mild annealing, yielding the formation of desorbing products (hydrogen, water) and of the metalated tetrapyrrole. We show here that the self-metalation of 2H-TPP molecules at the Pd(100) surface can be promoted already at room temperature by the presence of an oxygen gas phase at close-to-ambient conditions via an Eley-Rideal mechanism, resembling the asymmetric interactions available in enzymatic pockets, thus opening the way to novel routes to metalate organic frameworks at surfaces.
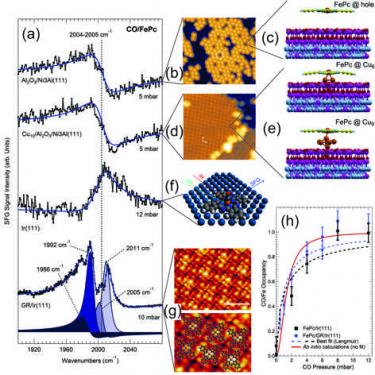 Tetrapyrroles at near-ambient pressure: porphyrins and phthalocyanines beyond the pressure gap [J. Phys. Mater. 2020]. Invited Review Paper. Many complex mechanisms underlying the fascinating functionalities provided by tetrapyrrolic macrocycles in biochemistry have been already unraveled. Light harvesting, molecular transport, and catalytic conversion are some of the processes performed by tetrapyrrole-based centers embedded in protein pockets. The main function is determined by the single atom species that is caged in the macrocycle, while a finer tuning (band gap, chemical selectivity etc) is granted by the geometric and electronic structure of the tetrapyrrole, including its residues, and by the proximal and distal structures of the protein surroundings that exploit the molecular trans-effect and direct weak interactions, respectively. Hence, a scientific and technological challenge consists in the artificial replication of both structure and functionality of natural reaction centers in 2D ordered arrays at surfaces. Nano-architected 2D metalorganic frameworks can be indeed self-assembled under controlled conditions at supporting surfaces and, in the specific, porphyrin- and phthalocyaninebased systems have been widely investigated in ultra-high vacuum conditions by means of surface science approaches. Deep insight into the geometry, electronic structure, magnetic properties, ligand adsorption mechanisms, and light absorption has been obtained, with the strong experimental constraint of vacuum. Especially in the case of the interaction of tetrapyrroles with ligands, this limit represents a relevant gap with respect to both comparison with natural counterparts from the liquid environment and potential applicative views at both solid–liquid and solid–gas interfaces. Thus, a step forward in the direction of near-ambient pressure is strongly necessary, while maintaining the atomiclevel detail characterization accuracy. Nowadays this becomes feasible by exploiting state-of-the-art experimental techniques, in combination with computational simulations. This review focusses on the latest advances in this direction.
Tetrapyrroles at near-ambient pressure: porphyrins and phthalocyanines beyond the pressure gap [J. Phys. Mater. 2020]. Invited Review Paper. Many complex mechanisms underlying the fascinating functionalities provided by tetrapyrrolic macrocycles in biochemistry have been already unraveled. Light harvesting, molecular transport, and catalytic conversion are some of the processes performed by tetrapyrrole-based centers embedded in protein pockets. The main function is determined by the single atom species that is caged in the macrocycle, while a finer tuning (band gap, chemical selectivity etc) is granted by the geometric and electronic structure of the tetrapyrrole, including its residues, and by the proximal and distal structures of the protein surroundings that exploit the molecular trans-effect and direct weak interactions, respectively. Hence, a scientific and technological challenge consists in the artificial replication of both structure and functionality of natural reaction centers in 2D ordered arrays at surfaces. Nano-architected 2D metalorganic frameworks can be indeed self-assembled under controlled conditions at supporting surfaces and, in the specific, porphyrin- and phthalocyaninebased systems have been widely investigated in ultra-high vacuum conditions by means of surface science approaches. Deep insight into the geometry, electronic structure, magnetic properties, ligand adsorption mechanisms, and light absorption has been obtained, with the strong experimental constraint of vacuum. Especially in the case of the interaction of tetrapyrroles with ligands, this limit represents a relevant gap with respect to both comparison with natural counterparts from the liquid environment and potential applicative views at both solid–liquid and solid–gas interfaces. Thus, a step forward in the direction of near-ambient pressure is strongly necessary, while maintaining the atomiclevel detail characterization accuracy. Nowadays this becomes feasible by exploiting state-of-the-art experimental techniques, in combination with computational simulations. This review focusses on the latest advances in this direction.
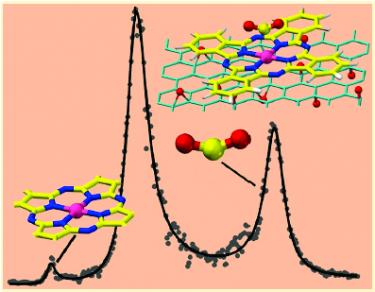 Learning from Nature: Charge Transfer and Carbon Dioxide Activation at Single, Biomimetic Fe Sites in Tetrapyrroles on Graphene [J. Phys. Chem. C 2019]. Nature determines selectivity and activity in biological reactive centers, based on single metal atom macrocycles, by properly tuning the primary coordination sphere and the surrounding protein scaffold. In a biomimetic approach, we show that activation of carbon dioxide at a 2D crystal of phthalocyanines supported by graphene can be controlled by chemical tuning of the position of the Dirac cones of the support through oxygen adsorption. The room temperature stabilization of the CO2−Fe chemical bond, detected in situ and confirmed by computational density-functional theory simulations, is obtained by governing the charge transfer across the graphene−metallorganic layer interface upon oxidation of graphene at close-to-ambient conditions. In this way, we can turn a weakly binding site into a strong one in an artificial structure that mimics many features of complex biological systems.
Learning from Nature: Charge Transfer and Carbon Dioxide Activation at Single, Biomimetic Fe Sites in Tetrapyrroles on Graphene [J. Phys. Chem. C 2019]. Nature determines selectivity and activity in biological reactive centers, based on single metal atom macrocycles, by properly tuning the primary coordination sphere and the surrounding protein scaffold. In a biomimetic approach, we show that activation of carbon dioxide at a 2D crystal of phthalocyanines supported by graphene can be controlled by chemical tuning of the position of the Dirac cones of the support through oxygen adsorption. The room temperature stabilization of the CO2−Fe chemical bond, detected in situ and confirmed by computational density-functional theory simulations, is obtained by governing the charge transfer across the graphene−metallorganic layer interface upon oxidation of graphene at close-to-ambient conditions. In this way, we can turn a weakly binding site into a strong one in an artificial structure that mimics many features of complex biological systems.
 Vibrational fingerprint of localized excitons in a two-dimensional metal-organic crystal [Nature Communications 2018]. Long-lived excitons formed upon visible light absorption play an essential role in photovoltaics, photocatalysis, and even in high-density information storage. Here, we describe a self-assembled two-dimensional metal-organic crystal, composed of graphene-supported macrocycles, each hosting a single FeN4 center, where a single carbon monoxide molecule can adsorb. In this heme-like biomimetic model system, excitons are generated by visible laser light upon a spin transition associated with the layer 2D crystallinity, and are simultaneously detected via the carbon monoxide ligand stretching mode at room temperature and near-ambient pressure. The proposed mechanism is supported by the results of infrared and time-resolved pump-probe spectroscopies, and by ab initio theoretical methods, opening a path towards the handling of exciton dynamics on 2D biomimetic crystals.
Vibrational fingerprint of localized excitons in a two-dimensional metal-organic crystal [Nature Communications 2018]. Long-lived excitons formed upon visible light absorption play an essential role in photovoltaics, photocatalysis, and even in high-density information storage. Here, we describe a self-assembled two-dimensional metal-organic crystal, composed of graphene-supported macrocycles, each hosting a single FeN4 center, where a single carbon monoxide molecule can adsorb. In this heme-like biomimetic model system, excitons are generated by visible laser light upon a spin transition associated with the layer 2D crystallinity, and are simultaneously detected via the carbon monoxide ligand stretching mode at room temperature and near-ambient pressure. The proposed mechanism is supported by the results of infrared and time-resolved pump-probe spectroscopies, and by ab initio theoretical methods, opening a path towards the handling of exciton dynamics on 2D biomimetic crystals.
 Substrate- to Laterally-Driven Self-Assembly Steered by Cu Nanoclusters: The Case of FePcs on an Ultrathin Alumina Film [ACS Nano 2018]. We show that, for the formation of a metallorganic monolayer, it is possible to artificially divert from substrate- to laterally-driven self-assembly mechanisms
Substrate- to Laterally-Driven Self-Assembly Steered by Cu Nanoclusters: The Case of FePcs on an Ultrathin Alumina Film [ACS Nano 2018]. We show that, for the formation of a metallorganic monolayer, it is possible to artificially divert from substrate- to laterally-driven self-assembly mechanisms
by properly tailoring the corrugation of the potential energy surface of the growth template. By exploiting the capability of an ultrathin alumina film to host metallic nanoparticle seeds, we tune the symmetry of a iron phthalocyanine (FePc) two-dimensional crystal, thus showing that it is possible to switch from trans to lateral dominating interactions in the controlled growth of an organic/inorganic heterostack. Finally, by selecting the size of the metallic clusters, we can also control the FePc−metal interaction strength.
 An in situ near-ambient pressure X-ray photoelectron spectroscopy study of CO2 reduction at Cu in a SOE cell [J. Elechem. 2017]. The cathodic behavior of a model solid oxide electrolysis cell (SOEC) has been studied by means of near-ambient pressure (NAP) X-ray Photoelectron Spectroscopy (XPS) and Near Edge X-ray Absorption Fine Structure Spectroscopy (NEXAFS), aiming at shedding light on the specific role of the metallic component in a class of cermets used as electrodes. The focus is on the surface chemistry and catalytic role of Cu, the increasingly popular metallic component in electrodes used in CO2 electrolysis and CO2/H2O co-electrolysis. The NAP-XPS and NEXAFS results, obtained in situ and operando conditions and under electrochemical control, have provided important insights about the evolution of the chemical composition of the Cu surface. We have found that in dry CO2 ambient carbon deposits are scavenged at low cathodic potential by the oxidising action of nascent O, while at high cathodic polarisations C grows due to activation of CO reduction. Instead, in CO2/H2O mixtures, surface deposit of C is steady over the whole investigated potential range. The presence of adsorbed CO has also been detected during electrolysis of CO2/H2O mixtures, while no CO is found in pure CO2 ambient.
An in situ near-ambient pressure X-ray photoelectron spectroscopy study of CO2 reduction at Cu in a SOE cell [J. Elechem. 2017]. The cathodic behavior of a model solid oxide electrolysis cell (SOEC) has been studied by means of near-ambient pressure (NAP) X-ray Photoelectron Spectroscopy (XPS) and Near Edge X-ray Absorption Fine Structure Spectroscopy (NEXAFS), aiming at shedding light on the specific role of the metallic component in a class of cermets used as electrodes. The focus is on the surface chemistry and catalytic role of Cu, the increasingly popular metallic component in electrodes used in CO2 electrolysis and CO2/H2O co-electrolysis. The NAP-XPS and NEXAFS results, obtained in situ and operando conditions and under electrochemical control, have provided important insights about the evolution of the chemical composition of the Cu surface. We have found that in dry CO2 ambient carbon deposits are scavenged at low cathodic potential by the oxidising action of nascent O, while at high cathodic polarisations C grows due to activation of CO reduction. Instead, in CO2/H2O mixtures, surface deposit of C is steady over the whole investigated potential range. The presence of adsorbed CO has also been detected during electrolysis of CO2/H2O mixtures, while no CO is found in pure CO2 ambient.
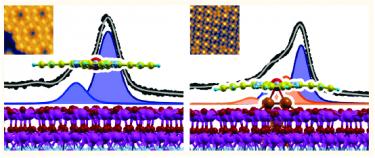
 Experimental and Theoretical Investigation of the Restructuring Process Induced by CO at Near Ambient Pressure: Pt Nanoclusters on Graphene/Ir(111) [ACS Nano 2017]. The adsorption of CO on Pt nanoclusters grown in a regular array on a template provided by the graphene/Ir(111) Moiré was investigated by means of infrared-visible sum frequency generation vibronic spectroscopy, scanning tunneling microscopy, X-ray photoelectron spectroscopy from ultrahigh vacuum to near-ambient pressure, and ab initio simulations. Both terminally and bridge bonded CO species populate nonequivalent sites of the clusters, spanning from first to second-layer terraces to borders and edges, depending on the particle size and morphology and on the adsorption conditions. By combining experimental information and the results of the simulations, we observe a significant restructuring of the clusters. Additionally, above room temperature and at 0.1 mbar, Pt clusters catalyze the spillover of CO to the underlying graphene/Ir(111) interface.
Experimental and Theoretical Investigation of the Restructuring Process Induced by CO at Near Ambient Pressure: Pt Nanoclusters on Graphene/Ir(111) [ACS Nano 2017]. The adsorption of CO on Pt nanoclusters grown in a regular array on a template provided by the graphene/Ir(111) Moiré was investigated by means of infrared-visible sum frequency generation vibronic spectroscopy, scanning tunneling microscopy, X-ray photoelectron spectroscopy from ultrahigh vacuum to near-ambient pressure, and ab initio simulations. Both terminally and bridge bonded CO species populate nonequivalent sites of the clusters, spanning from first to second-layer terraces to borders and edges, depending on the particle size and morphology and on the adsorption conditions. By combining experimental information and the results of the simulations, we observe a significant restructuring of the clusters. Additionally, above room temperature and at 0.1 mbar, Pt clusters catalyze the spillover of CO to the underlying graphene/Ir(111) interface.
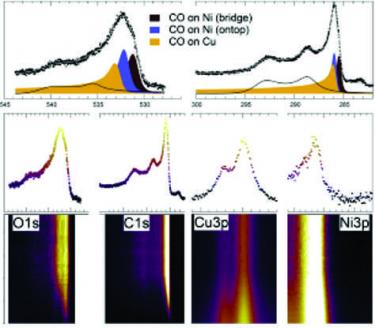 Tunability of the CO adsorption energy on a Ni/Cu surface: site change and coverage effects [J. Chem. Phys. 2017]. The adsorption energy of carbon monoxide on Ni ad-islands and ultra-thin films grown on the Cu(110) surface can be finely tuned via a complex interplay among diffusion, site change mechanisms, and coverage effects. The observed features of CO desorption can be explained in terms of migration of CO molecules from Cu to Ni islands, competition between bridge and on-top adsorption sites, and repulsive lateral adsorbate-adsorbate interactions. While the CO adsorption energy on clean Cu(110) is of the order of 0.5 eV, Ni-alloying allows for its controlled, continuous tunability in the 0.98-1.15 eV range with Ni coverage. Since CO is a fundamental reactant and intermediate in many heterogeneous catalytic (electro)-conversion reactions, insight into these aspects with atomic level detail provides useful information to potentially drive applicative developments. The tunability range of the CO adsorption energy that we measure is compatible with the already observed tuning of conversion rates by Ni doping of Cu single crystal catalysts for methanol synthesis from a CO2, CO, and H2 stream under ambient pressure conditions.
Tunability of the CO adsorption energy on a Ni/Cu surface: site change and coverage effects [J. Chem. Phys. 2017]. The adsorption energy of carbon monoxide on Ni ad-islands and ultra-thin films grown on the Cu(110) surface can be finely tuned via a complex interplay among diffusion, site change mechanisms, and coverage effects. The observed features of CO desorption can be explained in terms of migration of CO molecules from Cu to Ni islands, competition between bridge and on-top adsorption sites, and repulsive lateral adsorbate-adsorbate interactions. While the CO adsorption energy on clean Cu(110) is of the order of 0.5 eV, Ni-alloying allows for its controlled, continuous tunability in the 0.98-1.15 eV range with Ni coverage. Since CO is a fundamental reactant and intermediate in many heterogeneous catalytic (electro)-conversion reactions, insight into these aspects with atomic level detail provides useful information to potentially drive applicative developments. The tunability range of the CO adsorption energy that we measure is compatible with the already observed tuning of conversion rates by Ni doping of Cu single crystal catalysts for methanol synthesis from a CO2, CO, and H2 stream under ambient pressure conditions.
 Nanoscale control of metal clusters on templating supports [Elsevier]. Finite-size effects of nanometric metallic particles can be exploited to obtain tailored and novel electronic and chemical properties. At variance with traditional metal-dispersed supported heterogeneous catalysts widely employed for today’s applicative purposes, a thorough control of size, shape and alloying, together with a careful choice of the templating support, may yield to a bottom-up approach to the design of novel materials. However, challenges show up when dealing with realistic systems. Indeed, proper recipes to grow well-ordered arrays of equally-sized supported clusters are not straightforward, even if self-assembly and seeding strategies are adopted or if cluster sources are employed. In addition, with respect to model ultra-high vacuum conditions under which these systems are generally investigated, realistic working conditions require thermal stability, in order to avoid sintering effects, while interaction with an ambient pressure gas phase may influence the shape, composition, and chemical state of the nanoclusters yielding restructuring, poisoning, and deactivation. In this essay we review up-to-date experimental and theoretical approaches to investigate these issues, providing examples of selected systems, and outline a perspective direction to progressively close the material and pressure gaps for the effective transferability of modern surface science modeling to potentially applicative conditions.
Nanoscale control of metal clusters on templating supports [Elsevier]. Finite-size effects of nanometric metallic particles can be exploited to obtain tailored and novel electronic and chemical properties. At variance with traditional metal-dispersed supported heterogeneous catalysts widely employed for today’s applicative purposes, a thorough control of size, shape and alloying, together with a careful choice of the templating support, may yield to a bottom-up approach to the design of novel materials. However, challenges show up when dealing with realistic systems. Indeed, proper recipes to grow well-ordered arrays of equally-sized supported clusters are not straightforward, even if self-assembly and seeding strategies are adopted or if cluster sources are employed. In addition, with respect to model ultra-high vacuum conditions under which these systems are generally investigated, realistic working conditions require thermal stability, in order to avoid sintering effects, while interaction with an ambient pressure gas phase may influence the shape, composition, and chemical state of the nanoclusters yielding restructuring, poisoning, and deactivation. In this essay we review up-to-date experimental and theoretical approaches to investigate these issues, providing examples of selected systems, and outline a perspective direction to progressively close the material and pressure gaps for the effective transferability of modern surface science modeling to potentially applicative conditions.
 Room Temperature Carbonylation of Iron-Phthalocyanines Adsorbed on a Single Crystal Metal Surface: an in Situ SFG Investigation at Near-Ambient Pressure [J. Phys. Chem. C 2016]. We provide the experimental spectroscopic evidence for the room temperature carbonylation, at equilibrium with the carbon monoxide gas phase, of iron phthalocyanines adsorbed on the (111) termination of the iridium single crystal. The adsorption process occurs at CO pressures above the mbar yield. Interestingly, heme and heme-like catalytic, carrier, and enzymatic biomolecules interact with the gaseous reactants at partial pressures that are similar to the values that we observed on this model system, a 2D layer of single-atom metallorganic catalysts pre-assembled under ultra-high vacuum conditions. A simple Langmuir description of the adsorption mechanism yields a CO-Fe binding energy of 0.1-0.3 eV, depending on the assumptions about the pre-exponential factor. The internal vibrational structure of the adsorbed iron phthalocyanines is also investigated.
Room Temperature Carbonylation of Iron-Phthalocyanines Adsorbed on a Single Crystal Metal Surface: an in Situ SFG Investigation at Near-Ambient Pressure [J. Phys. Chem. C 2016]. We provide the experimental spectroscopic evidence for the room temperature carbonylation, at equilibrium with the carbon monoxide gas phase, of iron phthalocyanines adsorbed on the (111) termination of the iridium single crystal. The adsorption process occurs at CO pressures above the mbar yield. Interestingly, heme and heme-like catalytic, carrier, and enzymatic biomolecules interact with the gaseous reactants at partial pressures that are similar to the values that we observed on this model system, a 2D layer of single-atom metallorganic catalysts pre-assembled under ultra-high vacuum conditions. A simple Langmuir description of the adsorption mechanism yields a CO-Fe binding energy of 0.1-0.3 eV, depending on the assumptions about the pre-exponential factor. The internal vibrational structure of the adsorbed iron phthalocyanines is also investigated.
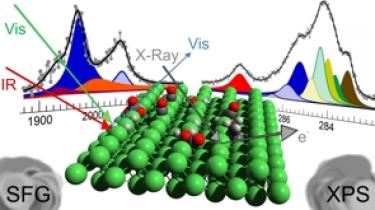 Reverse Water−Gas Shift or Sabatier Methanation on Ni(110) [JACS 2016]. The interaction of CO, CO2, CO + H2, CO2 + H2, and CO + CO2 + H2 with the nickel (110) single crystal termination has been investigated at 10−1 mbar in situ as a function of the surface temperature in the 300−525 K range by means of infrared-visible sum frequency generation (IR−vis SFG) vibrational spectroscopy and by near-ambient pressure X-ray photoelectron spectroscopy (NAP-XPS). Several stable surface species have been observed and identified. Besides atomic carbon and precursors for graphenic C phases, five nonequivalent CO species have been distinguished, evidencing the role of coadsorption effects with H and C atoms, of H-induced activation of CO, and of surface reconstruction. At low temperature, carbonate species produced by the interaction of CO2 with atomic oxygen, which stems from the dissociation of CO2 into CO + O, are found on the surface. A metastable activated CO2 − species is also detected, being at the same time a precursor state toward dissociation into CO and O in the reverse water−gas shift mechanism and a reactive species that undergoes direct conversion in the Sabatier methanation process. Finally, the stability of ethylidyne is deduced on the basis of our spectroscopic observations.
Reverse Water−Gas Shift or Sabatier Methanation on Ni(110) [JACS 2016]. The interaction of CO, CO2, CO + H2, CO2 + H2, and CO + CO2 + H2 with the nickel (110) single crystal termination has been investigated at 10−1 mbar in situ as a function of the surface temperature in the 300−525 K range by means of infrared-visible sum frequency generation (IR−vis SFG) vibrational spectroscopy and by near-ambient pressure X-ray photoelectron spectroscopy (NAP-XPS). Several stable surface species have been observed and identified. Besides atomic carbon and precursors for graphenic C phases, five nonequivalent CO species have been distinguished, evidencing the role of coadsorption effects with H and C atoms, of H-induced activation of CO, and of surface reconstruction. At low temperature, carbonate species produced by the interaction of CO2 with atomic oxygen, which stems from the dissociation of CO2 into CO + O, are found on the surface. A metastable activated CO2 − species is also detected, being at the same time a precursor state toward dissociation into CO and O in the reverse water−gas shift mechanism and a reactive species that undergoes direct conversion in the Sabatier methanation process. Finally, the stability of ethylidyne is deduced on the basis of our spectroscopic observations.
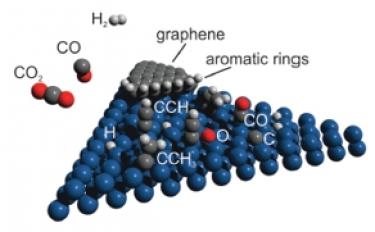 Carbon dioxide reduction on Ir(111) [PCCP 2016]. Stable hydrocarbon surface species in the carbon dioxide hydrogenation reaction on Ir(111) were identified by means of infrared-visible sum-frequency generation vibrational spectroscopy and X-ray photoelectron spectroscopy under near-ambient pressure conditions (0.1 mbar). By introducing gas phase binary and ternary mixtures of CO2, CO, and H2 into the reaction chamber, stable ethylidyne and ethynyl species were found at the metal surface above 425 K, in remarkable analogy with that observed during the ethylene decomposition process yielding graphene. In addition, upon increasing temperature (up to 600 K depending on the reaction conditions), vibrational and electronic spectroscopic fingerprints appeared that could be attributed to the nucleation of aromatic hydrocarbons at the edge of metastable graphenic clusters interacting with the metal surface.
Carbon dioxide reduction on Ir(111) [PCCP 2016]. Stable hydrocarbon surface species in the carbon dioxide hydrogenation reaction on Ir(111) were identified by means of infrared-visible sum-frequency generation vibrational spectroscopy and X-ray photoelectron spectroscopy under near-ambient pressure conditions (0.1 mbar). By introducing gas phase binary and ternary mixtures of CO2, CO, and H2 into the reaction chamber, stable ethylidyne and ethynyl species were found at the metal surface above 425 K, in remarkable analogy with that observed during the ethylene decomposition process yielding graphene. In addition, upon increasing temperature (up to 600 K depending on the reaction conditions), vibrational and electronic spectroscopic fingerprints appeared that could be attributed to the nucleation of aromatic hydrocarbons at the edge of metastable graphenic clusters interacting with the metal surface.
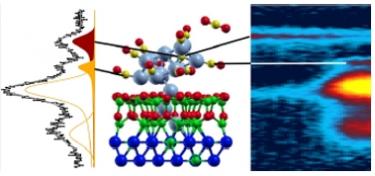 CO on Supported Cu Nanoclusters [ACS Catal. 2015]. The interaction of carbon monoxide with an ordered array of copper nanoclusters was investigated under ultrahigh vacuum conditions by means of in situ X-ray photoelectron spectroscopy in combination with density functional theory calculations. The Cu clusters were supported on an alumina template grown on the Ni3Al(111) termination. Adsorption and dissociation of carbon monoxide occur at the copper clusters, yielding accumulation of carbidic carbon at the metal particles through the Boudouard process. The involved mechanisms are investigated at the atomic level, unveiling the effects of cluster finite size, reconstruction, support, and of local CO coverage. It is found that the high coverage of CO at the cluster surface, which considerably exceeds that achievable on single crystal surfaces, facilitates the metal restructuring and the reaction, yielding carbon incorporation into the bulk of the particles.
CO on Supported Cu Nanoclusters [ACS Catal. 2015]. The interaction of carbon monoxide with an ordered array of copper nanoclusters was investigated under ultrahigh vacuum conditions by means of in situ X-ray photoelectron spectroscopy in combination with density functional theory calculations. The Cu clusters were supported on an alumina template grown on the Ni3Al(111) termination. Adsorption and dissociation of carbon monoxide occur at the copper clusters, yielding accumulation of carbidic carbon at the metal particles through the Boudouard process. The involved mechanisms are investigated at the atomic level, unveiling the effects of cluster finite size, reconstruction, support, and of local CO coverage. It is found that the high coverage of CO at the cluster surface, which considerably exceeds that achievable on single crystal surfaces, facilitates the metal restructuring and the reaction, yielding carbon incorporation into the bulk of the particles.
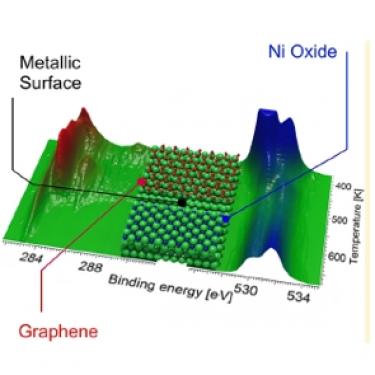 Reactivity of Carbon Dioxide on Nickel [J. Phys. Chem. Lett. 2014]. The catalytic conversion of carbon dioxide to synthetic fuels and other valuable chemicals is an issue of global environmental and economic impact. In this report we show by means of X-ray photoelectron spectroscopy in the mbar range that, on a Ni surface, the reduction of carbon dioxide is indirectly governed by the CO chemistry. While the growth of graphene and the carbide-graphene conversion can be controlled by selecting the reaction temperature, oxygen is mainly removed by CO, since oxygen reduction by hydrogen is a slow process on Ni. Even though there is still a consistent pressure gap with respect to industrial reaction conditions, the observed phenomena provide a plausible interpretation of the behavior of Ni/Cu based catalysts for CO2 conversion and account for a possible role of CO in the methanol synthesis process.
Reactivity of Carbon Dioxide on Nickel [J. Phys. Chem. Lett. 2014]. The catalytic conversion of carbon dioxide to synthetic fuels and other valuable chemicals is an issue of global environmental and economic impact. In this report we show by means of X-ray photoelectron spectroscopy in the mbar range that, on a Ni surface, the reduction of carbon dioxide is indirectly governed by the CO chemistry. While the growth of graphene and the carbide-graphene conversion can be controlled by selecting the reaction temperature, oxygen is mainly removed by CO, since oxygen reduction by hydrogen is a slow process on Ni. Even though there is still a consistent pressure gap with respect to industrial reaction conditions, the observed phenomena provide a plausible interpretation of the behavior of Ni/Cu based catalysts for CO2 conversion and account for a possible role of CO in the methanol synthesis process.
Research Group.
- Group leader: Erik Vesselli; Associate Professor at the Physics Department, UniTs;
- Pietro Biasin, PhD student in Physics at UniTs;
- Francesco Armillotta; PhD student in Physics at UniTs;
- Stefania Baronio, PhD student in Physics at UniTs;
- Students: Elena Ghidorsi, Paola Mantegazza, Danilo Comini;
Former students/staff.
Research staff
- Matus Stredansky, PostDoc;
PhD Students
- Tommaso Fontanot, PhD Thesis Title: "Experimental characterization of surfaces and metal-plymer heterostacks of optical systems for the automotive sector"
- Manuel Corva, PhD Thesis in Nanotechnology, Title: "Experimental modeling of nanostructured and single metal atom supported catalysts at close-to-ambient conditions"
- Mario Filiasi, PhD Thesis in Physics, Title: “Applications of large deviations theory and statistical inference to financial time series”
Students
AA 2020-2021
- Stefania Baronio: Master thesis "Mono- and bi-metallic organic layers on graphene: electronic and vibronic characterization by means of pump-probe and non-linear spectroscopies";
AA 2019-2020
- Alessandro Namar: Bachelor thesis "Caratterizzazione spettroscopica di un’eterostruttura metallorganica mediante analisi dei livelli di core: (Co-Fe)TPyP su grafene";
- Leonardo Toffolin: Bachelor thesis "Spettroscopia ottica non lineare: sviluppo di uno strumento per l'analisi dei dati ottenuti mediante il processo di generazione di frequenza somma";
- Pietro Biasin: Master thesis "NEXAFS characterization of a metalorganic heterostructure: (Co-Fe)TPyP on graphene";
- Carlotta Marchiori: Master thesis "Through-Transmission Laser Welding of Thermoplastics at IR wavelengths: a Finite Element Simulation Approach";
- Antonio Annese: Master thesis "Caratterizzazione spettroscopica dei livelli di core dell’eterostruttura metallorganica formata da (Co-Fe)TPyP su grafene";
AA 2018-2019
- Mattia Vadori: "Ottimizzazione di sistemi ottici non-imaging";
- Michele Berti: Bachelor thesis "Analisi e sviluppo di uno strumento per il pricing di un portafoglio finanziario diversificato";
- Alex Pividori: Master thesis "Interaction of molecular oxygen with cobalt tetrapyridylporphyrins: an ab initio investigation";
- Francesco Armillotta: Master thesis "Metalation and reactivity of self-assembled tetrapyrroles at surfaces";
- Enrico D'Incecco: Master thesis "Self-metalation of tetraphenyl porphyrins on a surface: in situ investigation of the role of the gas phase at near-ambient pressure";
AA 2017-2018
- Francesca Rossi: Master thesis "Design and fabrication of a Zn-Air battery prototype and in situ characterization of the Zn electrodeposition process";
- Stefania Moro: Master thesis "Vibronic characterization of 2D systems: internal modes and chemisorption of small molecules";
- Anita Previdi: Master thesis "In situ and operando spectroscopic characterization of model electrochemical cells";
AA 2016-2017
- Erika Tomsic: Master thesis "Interaction of iron phthalocyanines with alumina-supported copper clusters: a model for a zero-dimensional metalorganic alloy catalyst";
- Matteo Rinaldi: Master thesis "Adsorption of CO and CO2 on iron phthalocyanines: an ab initio investigation of structural, electronic, and vibrational properties";
AA 2015-2016
- Nicola Podda: Master thesis "Reactivity of platinum clusters investigated by means of vibronic spectroscopy and tunneling microscopy"
- Alberto Ferrari: Master thesis "Ab-initio investigation of reactivity, electronic and vibrational properties of iron phthalocyanines"
AA 2014-2015
- Manuel Corva: Master thesis “CO and CO2 interaction owith iridium and iridium-supported model catalysts: a near-ambient pressure spectroscopic investigation”
- Giulia Leghissa: Bachelor thesis “Analisi della fase gassosa: modifica di una cella ad alta pressione per spetrtoscopia SFG”
AA 2013-2014
- Laura Cataldi: Bachelor thesis “Caratterizzazione del Sistema DMSO/Au(111) mediante spettroscopia vibrazionale SFG”
- Matteo Roiaz: Master thesis “Heterogeneous catalytic reduction of CO2: SFG vibrational spectroscopy at near ambient pressure and in liquid environment”
- Paolo Zucchiatti: Master thesis “Discriminazione dei contributi spettrali del DNA e dell’RNA nel profilo vibrazionale infrarosso di cellule eucariotiche”
AA 2012-2013
- Antonio Tavano: Bachelor thesis “Modello di Merton jump diffusion: analisi della convergenza e studio della funzione densità di probabilità”
- Enrico Monachino: Master thesis “Catalytic reduction of carbon dioxide on a nickel single crystal: an in situ investigation”
- Stefano Speranzon: Master thesis “Analisi ed implementazione di un modello stocastico per la valutazione quantitative del rischio di default”
Collaborations.
- Institute of Materials Chemistry, Technische Universitaet Wien IMC-TUW (Vienna, A)
- CNR-ICCOM (Firenze, IT)
- CNR-IOM (Trieste, IT)
- DEMOCRITOS (Trieste, IT)
- Università del Salento, Dipartimento di Ingegneria dell’Innovazione (Lecce, IT)
- Dipartimento di Energia, Politecnico di Milano (Milano, IT)
- Chair of Interface Research and Catalysis, Department of Chemistry and Pharmacy, FAU (Erlangen, DE)
- FHI Fritz Haber Institute, Department of Inorganic Chemistry (Berlin, DE)
- ICTP International Center for Theoretical Physics (Trieste, IT)
- Elettra Sincrotrone Trieste ScpA (Trieste; IT)
- Charles University (Prague, CZ)
- List SpA
- ModeFinance Srl
- Automotive Lighting - Magneti Marelli
Funding and contributors.
- UniTs through FRA projects 2012 and 2016
- MIUR FIRB2010 RBFR10J4H7
- MIUR PRIN2017
- Beneficentia Stiftung
- Fondazione Casali
- Consorzio dei Dipartimenti di Fisica dell’Università degli Studi di Trieste
- Università degli Studi di Trieste (progetti FRA)
- Automotive Lighting - Magneti Marelli
Recent Relevant Publications.
2021
- T. Fontanot, S. Donato, F. Di Lillo, D. Dreossi, L. Rigon, R. Longo, S. Dal Zilio, R. Ciancio, S. Paroni, and E. Vesselli, “On the origin of surface and interface defects associated with the growth of Al‐coated thermoplastic heterostacks”: Surf. Int. Analysis 53 (2021) 350, DOI: 10.1002/sia.6923.
- V. Pramhaas, M. Roiaz, N. Bosio, M. Corva, C. Rameshan, E. Vesselli, H. Grönbeck, and G. Rupprechter, “Interplay between CO disproportionation and oxidation: on the origin of the CO reaction onset on ALD-grown Pt/ZrO2 model catalysts”: ACS Catal. 11 (2021) 208, DOI: 10.1021/acscatal.0c03974.
- E.Vesselli, “Stabilization and activation of molecular oxygen at biomimetic tetrapyrroles on surfaces: from UHV to near-ambient pressure”: Nanoscale Adv, 3 (2021) 1319, DOI: 10.1039/D0NA00827C.
- H.M. Sturmeit, I. Cojocariu, A. Windischbacher, P. Puschnig, C. Piamonteze, M. Jugovac, A. Sala, G. Comelli, A. Cossaro, A. Verdini, L. Floreano, M. Stredansky, E. Vesselli, C. Hohner, M. Kettner, J. Libuda, C.M. Schneider, G. Zamborlini, M. Cinchetti, and V. Feyer, “Room-temperature on-spin-switching and tuning in a porphyrin-based multifunctional interface”: Small 17 (2021) 2104779, DOI: 10.1002/smll.202104779.
- F. Armillotta, E. D’Incecco, M. Corva, M. Stredansky, J.-J. Gallet, F. Bournel, A. Goldoni, A. Morgante, E. Vesselli, and A. Verdini, “Room temperature self-metalation of porphyrins at the solid-gas interface”: Angewandte Chemie IE 60 (2021) 25988, DOI: 10.1002/anie.202111932.
2020
- I. Cojocariu, H.M. Sturmeit, G. Zamborlini, A. Cossaro, A. Verdini, L. Floreano, E. D’Incecco, M. Stredansky, E. Vesselli, M. Jugovac, M. Cinchetti, V. Feyer, and C. M. Schneider, “Evaluation of molecular orbital symmetry via oxygen-induced charge transfer quenching at a metal-organic interface”: Appl. Surf. Sci. 504 (2020) 144343, DOI: 10.1016/j.apsusc.2019.144343.
- E. Vesselli, “Tetrapyrroles at near-ambient pressure: porphyrins and phthalocyanines beyond the pressure gap”: J. Phys. Materials 3 (2020) 022002, DOI: 10.1088/2515-7639/ab7ab2.
- M. Stredansky, S. Moro, M. Corva, M. Jugovac, G. Zamborlini, V. Feyer, C.M. Schneider, I. Cojocariu, H.M. Sturmheit, M. Cinchetti, A. Verdini, A. Cossaro, L. Floreano, and E. Vesselli, “Vibronic Fingerprints of the Nickel Oxidation States in Surface-Supported Porphyrin Arrays”: J. Phys. Chem. C 214 (2020) 6297, DOI: 10.1021/acs.jpcc.0c01387.
- T. Fontanot, J. Audenaert, P. Hanselaer, I. Pecorari, V. Lughi, E. Vesselli, S. Paroni, and F. Leloup, “BRDF characterization of Al-coated thermoplastic polymer surfaces”: J. Coat. Technol. Res. XX (2020) YYY, DOI: 10.1007/s11998-020-00361-0.
- H. Sturmheit, I. Cojocariu, M. Jugovac, A. Cossaro, A. Verdini, L. Floreano, A. Sala, G. Comelli, S. Moro, M. Stredansky, M. Corva, E. Vesselli, P. Pusching, C.M. Schneider, V. Feyer, G. Zamborlini, and M. Cinchetti, “Molecular anchoring stabilizes low valence Ni(I)TPP on copper against thermally induced chemical changes”: J. Mater. Chem. C 8 (2020) 8876, DOI: 10.1039/D0TC00946F.
- F. Armillotta, A. Pividori, M. Stredansky, N. Seriani, and E. Vesselli, “Dioxygen at biomimetic single metal-atom sites: activation or stabilization? The case of CoTPyP/Au(111)”: Topics Catal., (2020), DOI: 10.1007/s11244-020-01333-9.
2019
- M. Corva, F. Mohamed, E. Tomsic, M. Rinaldi, C. Cepek, N. Seriani, M. Peressi, and (*) E. Vesselli, “Learning from Nature: Charge Transfer and Carbon Dioxide Activation at Single, Biomimetic Fe Sites in Tetrapyrroles on Graphene”: J. Phys. Chem. C 123 (2019) 3916, DOI: 10.1021/acs.jpcc.8b11871.
- T. Fontanot, D. Ermacora, G. Simnetti, S. Raducci, E. Vesselli, and S. Paroni, “An automatic visual inspection system to scan outer lenses of automotive rear lamps”: SPIE Proceedings 11056 (2019) 1105624, DOI: 10.1117/12.2525435.
- B. Bozzini, A. Previdi, M. Amati, M. Bevilacqua, G. Cordaro, M. Corva, A. Donazzi, G. Dotelli, L. Gregoratti, R. Pelosato, M. Vorokhta, and E. Vesselli, “In situ near-ambient pressure X-ray photoelectron spectroscopy discloses the surface composition of operating NdBaCo2O5+ solid oxide fuel cell cathodes”: J. Power Sources 436 (2019) 226815, DOI: 10.1016/j.jpowsour.2019.226815.
- F. Rossi, M. Bevilacqua, B. Busson, M. Corva, A. Tadjeddine, F. Vizza, E. Vesselli, and B. Bozzini, “An in situ IR-Vis Sum Frequency Generation Spectroscopy Study of Cyanide Adsorption during Zinc Electrodeposition”: J. Ele. Chem. 855 (2019) 113641, DOI: 10.1016/j.jelechem.2019.113641.
2018
- M. Corva, F. Mohamed, E. Tomsic, Z. Feng, T. Skala, G. Comelli, N. Seriani, M. Peressi, E. Vesselli, “Substrate- to laterally-driven self-assembly steered by Cu nanoclusters: the case of FePcs on an ultrathin alumina film”: ACS Nano 12 (2018) 10755, DOI: 10.1021/acsnano.8b05992.
- M. Corva, A. Ferrari, M. Rinaldi, Z. Feng, M. Roiaz, C. Rameshan, G. Rupprechter, R. Costantini, M. Dell’Angela, G. Pastore, G. Comelli, N. Seriani, and E. Vesselli, “Vibrational fingerprint of localized excitons in a 2D metalorganic crystal”: Nat. Commun. 9 (2018) 4703, DOI: 10.1038/s41467-018-07190-1.
2017
- N. Podda, M. Corva, F. Mohamed, Z. Feng, C. Dri, F. Dvorak, V. Matolin, G. Comelli, M. Peressi, and E. Vesselli, "Experimental and Theoretical Investigation of the Restructuring Process Induced by CO at Near Ambient Pressure: Pt Nanoclusters on Graphene/Ir(111)": ACS Nano 11 (2017) 1041, DOI: 10.1021/acsnano.6b07876.
- E. Vesselli, M. Peressi, “Nanoscale control of metal clusters on templating supports” in “Morphologcal, compositional, and shape control of materials for catalysis”, p. 285-315, vol. 177, Studies in Surface Science and Catalysis, P. Fornasiero and M. Cargnello eds., Elsevier (2017), ISBN: 9780128050903.
- B. Bozzini, M. Amati, C. Mele, A. Knop-Gericke, E. Vesselli, “An in situ near-ambient pressure X-ray Photoelectron Spectroscopy study of CO2 reduction at Cu in a SOE cell”: J. Elechem. 799 (2017) 17, DOI: 10.1016/j.jelechem.2017.05.011.
- E. Vesselli, M. Rizzi, S. Furlan, X. Duan, E. Monachino, C. Dri, A. Peronio, C. Africh, P. Lacovig, A. Baldereschi, G. Comelli, and M. Peressi, “Tunability of the CO adsorption energy on a Ni/Cu surface: site change and coverage effects.”: J. Chem. Phys. 146 (2017) 224707, DOI: 10.1063/1.4985657.
2016
- M. Corva, E. Vesselli, "Room Temperature Carbonylation of Iron-Phthalocyanines Adsorbed on a Single Crystal Metal Surface: an in Situ SFG Investigation at Near-Ambient Pressure": J. Phys. Chem. C 120 (2016) 22298, DOI: 10.1021/acs.jpcc.6b05356.
- M. Roiaz, E. Monachino, C. Dri, M. Greiner, A. Knop-Gericke, R. Schlögl, G. Comelli, and E. Vesselli, “Reverse water-gas shift or Sabatier methanation on Ni(110)? Stable surface species at near-ambient pressure”: J. Am. Chem. Soc. 138 (2016) 4146, DOI: 10.1021/jacs.5b13366.
- M. Corva, Z. Feng, C. Dri, F. Salvador, P. Bertoch, G. Comelli, and E. Vesselli, “Carbon dioxide reduction on Ir(111): stable hydrocarbon species at near-ambient pressure”: PhysChemChemPhys 18 (2016) 6763, DOI: 10.1039/c5cp07906c.
2015
- Z. Feng, S. Velari, A. Cossaro, C. Castellarin-Cudia, A. Verdini, E. Vesselli, C. Dri, M. Peressi, A. De Vita, and G. Comelli, “Trapping of charged gold adatoms by dimethyl sulfoxide on a gold surface”: ACS Nano 9 (2015) 8697, DOI: 10.1021/acsnano.5b02284.
- J.A. Olmos-Asar, Erik Vesselli, A. Baldereschi, and M. Peressi, “Towards optimal seeding for the synthesis of ordered nanoparticle arrays on alumina/Ni3Al(111)”: PhysChemChemPhys 17 (2015) 28154, DOI: 10.1039/c5cp00304k.
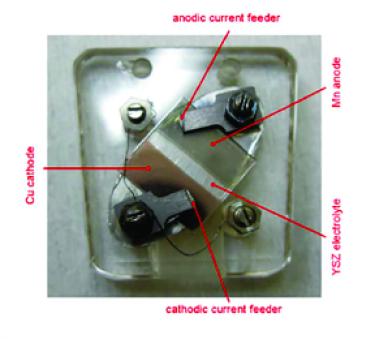
- B. Bozzini, M. Amati, P. Bocchetta, S. Dal Zilio, A. Knop-Gericke, E. Vesselli, and M. Kiskinova, “An in situ near-ambient pressure X-ray Photoelectron Spectroscopy study of Mn polarised anodically in a cell with solid oxide electrolyte”: Electrochimica Acta 174 (2015) 532, DOI: 10.1016/j.electacta.2015.05.173.
- J.A. Olmos-Asar, E. Monachino, C. Dri, A. Peronio, C. Africh, P. Lacovig, G. Comelli, A. Baldereschi, N. Peressi, and E. Vesselli, “CO on supported Cu nanoclusters: coverage and finite size contributions to the formation of carbide via the Boudouard process”: ACS Catal. 5 (2015) 2719, DOI: 10.1021/cs501361h.
2014
- J.A. Olmos-Asar, E. Vesselli, A. Baldereschi, M. Peressi, “Self-seeded nucleation of Cu nanoclusters on Al2O3/Ni3Al(111): an ab-inintio investigation”: PhysChemChemPhys 16 (2014) 23134; DOI: 10.1039/C4CP03271C.
- M. Filiasi, G. Livan, M. Marsili, E. Vesselli, and E. Zarinelli, “On the concentration of large deviations for fat tailed distributions, with application to financial data”: J. Stat. Phys. 9 (2014) 09030, DOI: 10.1088/1742-5468/2014/09/P09030.
- M. Peressi and E. Vesselli, “Combining surface science experiments and numerical simulations”: Il Nuovo Saggiatore 30 (2014) 4.
- E. Monachino, M. Greiner, A. Knop-Gericke, R. Schlögl, C. Dri, E. Vesselli, and G. Comelli, “Reactivity of carbon dioxide on nickel: role of CO in the competing interplay between oxygen and graphene”: J. Chem. Phys. Lett. 5 (2014) 1929, DOI: 10.1021/jz5007675.
- M. Bevilacqua, J. Filippi, A. Lavacchi, A. Marchionni, H.A. Miller, W. Oberhauser, E. Vesselli, and F. Vizza, “Energy saving in the conversion of CO2 and ethanol to fuels and raw chemicals by an electrolytic device”: En. Technol. 2 (2014) 522, DOI: 10.1002/ente.201402014.
2013
- H.A. Miller, F. Vizza, M. Bevilacqua, J. Filippi, A. Lavacchi, A. Marchionni, W. Oberhauser, S. Moneti, M. Marelli, E. Vesselli, and M. Innocenti, “Nanostructured Fe-Ag electrocatalysts for the oxygen reduction reaction in alkaline media”: J. Mat. Chem. A 1 (2013) 13337, DOI: 10.1039/c3ta12757e.
- E. Vesselli, E. Monachino, M. Rizzi, S. Furlan, X. Duan, C. Dri, A. Peronio, C. Africh, P. Lacovig, A. Baldereschi, G. Comelli, and M. Peressi, “Steering the chemistry of carbon oxides on a NiCu catalyst”: ACS Catal. 3 (2013) 1555, DOI: 10.1021/cs400327y.
2012
- M. Rizzi, S. Furlan, M. Peressi, A. Baldereschi, C. Dri, A. Peronio, C. Africh, P. Lacovig, E. Vesselli, and G. Comelli, “Tailoring bimetallic alloy surface properties by kinetic control of self-diffusion processes at the nanoscale”: J. Am. Chem. Soc. 134 (2012) 16827, DOI: 10.1021/ja307294p.